
Dr. Jianbo Liu
Professor and Acting EO of ChemistryCUNY Graduate Center and Queens College
News
- 2023. 03.
Jonathan and May were selected for the Graduate Center Dissertation Fellowship, winning $25k for FY24. - 2022. 08.
Liu Lab has partnered with the Air Force Research Laboratory via the Education Partnership Agreement (EPA). - 2022. 03.
Jessie and Jonathan are both awarded CUNY Doctoral Student Research Grant DSRG17.
Research
Current Projects
I. Investigate biochemical and biophysical interactions by gas-phase techniques
This project uses gas-phase ion-molecule reaction techniques, in conjunction with computational chemistry, to probe biophysical and biochemical processes. One of such research efforts is directed toward probing the oxidation, nitrosation and nitration reactions of biomolecules by reactive oxygen species (e.g. singlet oxygen) and reactive nitrogen species (•NOX), and oxidative post-translational modifications. These processes are associated with aging, mutations, diseases and radiotherapy/photodynamic therapy for cancer, and are also related to the photochemical transformations of atmospheric biomolecule aerosols. Most of such research is based on solution-phase experiments. The difficulty to separate, detect, and distinguish various short-lived reactive species in solution, together with the large number of coupled experimental variables (sensitizers, oxygen, solvent, pH, excitation source, etc.), makes the interpretation of reaction mechanism, kinetics and dynamics complicated.
Gas-phase techniques offer unique approaches that isolate reactants and interactions in an rarefied environment without solvent or other interferences. The experiment identifies early intermediates, quantifies transient species and products, and focuses on intrinsic reactivity of biomolecules. Resulting insights can then be extrapolated to solution models by incorporating hydration effects at two different levels: adding explicit water ligands to biomolecules in the gas phase, and forming aerosols and gas-phase reverse micelles containing water droplets.
Experimental Techniques: Biomolecular ions are generated by electrospray ionization (ESI). Ion-molecule reactions between biomolecular ions and neutral reactive species are carried out using guided-ion beam scattering methods. Gas-phase measurement includes reaction cross sections, product branching. Measurement of differential cross sections (i.e., recoil energy and angular distributions of products) is also possible. The instruments employed for this project include guided-ion beam tandem mass spectrometer, and general-purpose analytical spectrophotometers.
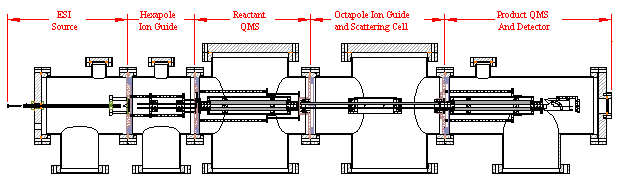
ESI guided-ion-beam tandem mass spectrometer
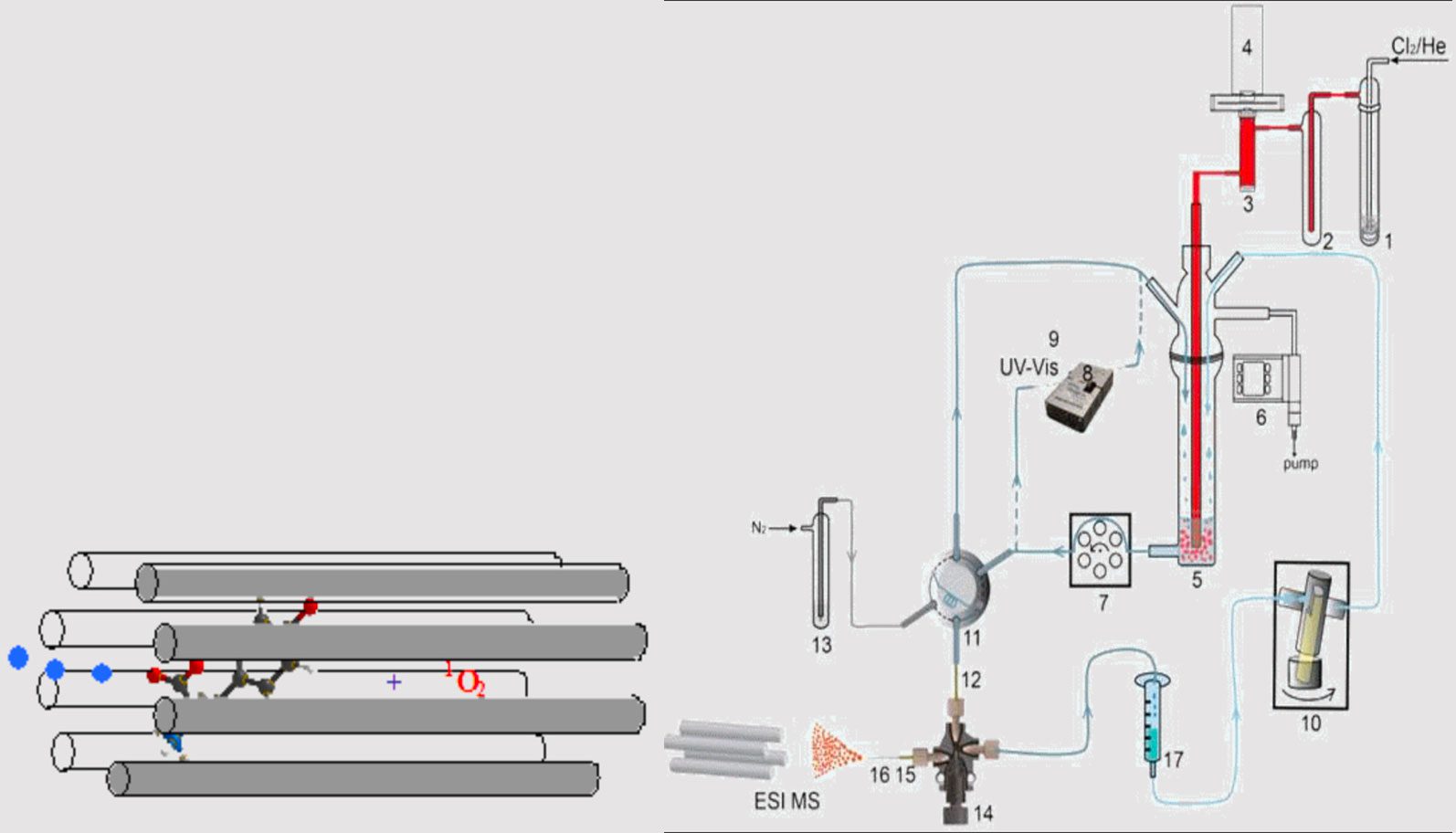
Gas-phase reaction may couple to solution chemistry
Computational Methods: In conjunction with experimental efforts, we use ab initio electronic structure calculations to map out reaction coordinates (i.e., intermediates, transition states, products, etc.). For systems with modest molecular weight, we perform direct dynamics trajectory simulations to examine dynamics. The combination of ion-beam scattering experiment with computational simulations allows us to probe the origin of experimentally measured reaction kinetics and dynamics, and help design experiment to target particular mechanistic or energetic issues.
II. Electrospray dynamics and kinetics of energetic ionic liquids

Multi-mode propulsion (MMP) systems that integrate chemical and electric propulsion are of current interest to the Space Force. Ionic liquids (ILs) are versatile materials defined as low melting salts that melt at or below 100 °C. ILs are often considered as energetic materials with applications ranging from tailored solvents for chemical synthesis, mixtures for explosives, and propellants for space propulsion. Due to their reduced toxicity and improved performance, ILs are also of great interest as a greener alternative to the highly toxic hydrazine monopropellant. In addition, they are candidates for electrospray propulsion owing to their inherent ionic nature, low vapor pressure and high electrical conductivities, yielding high specific impulse. ILs are therefore prime candidates for MMP.
This project is aimed at revealing the chemical reaction mechanisms and behaviors of these energetic materials to determine propellant utilization-metrics and to optimize thruster design and operation for MMP applications. Capitalizing on electrospray ionization guided-ion beam tandem mass spectrometry and ion-molecule reaction techniques, and augmented by molecular dynamics simulations, this research characterizes reaction structures, dynamics, and product branching ratios of the species present in the electrosprays of hydroxylammonium nitrate (HAN), 2-Hydroxyethylhydrazinium nitrate (HEHN) and other related ILs already fielded for space propulsion applications.
The broader impact of this work could lead to systems development/fielding of capabilities in the Cis-lunar space domain that support the MMP architecture by 12-31-2025.
III. Dynamics simulations of hypergolic reactions
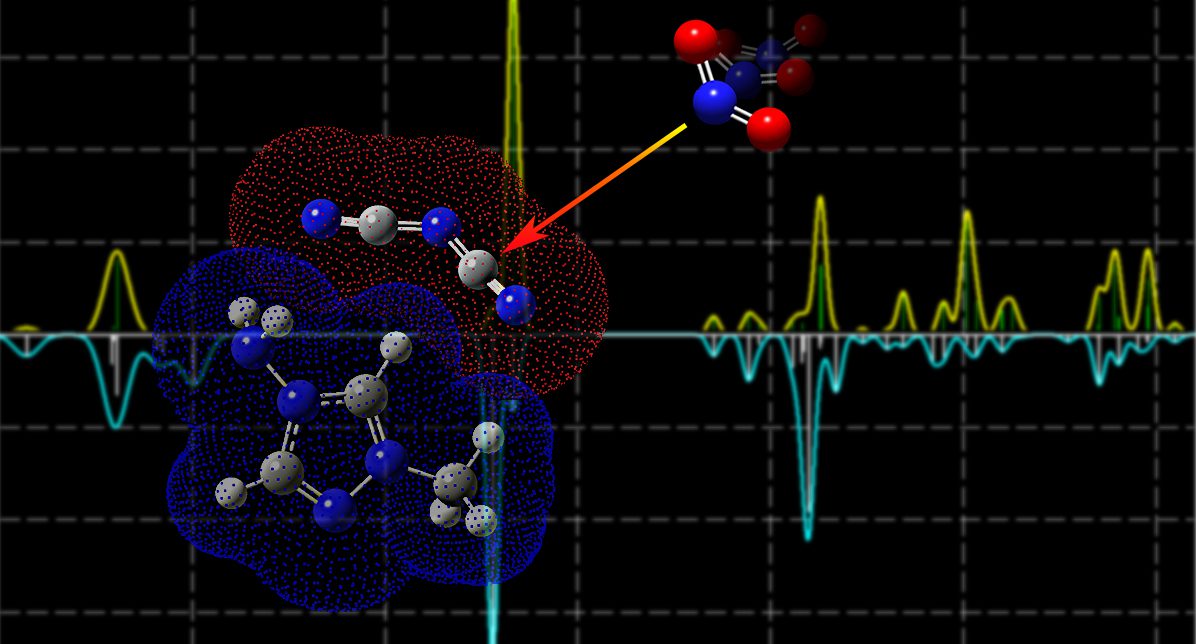
The designability of energetic ILs includes the choice of cation and anion within a single salt, which allows flexibility in customizing IL properties and performance for anticipated purposes. Cations (e.g. imidazolium and triazolium) affect physicochemical properties of fuels and ID times, while anions are associated with hypergolicity (spontaneous ignition with an oxidizer) and play a decisive role in the inducing stage of ignition.
Dicyanamide anion (N(CN)2–, abbreviated as DCA–) and dicyanoborohydride (H2B(CN)2–, abbreviated as DCBH–) are well known for their hypergolicity. The hypergolic process between fuel and oxidizer involves two stages: the low-temperature preignition phase where thermolytic and oxidative processes in the condensed phase begin to decompose both ILs and oxidizers, produce gaseous species and increase the temperature of the system; and the ignition and subsequently combustion once the temperature and concentration of key species reach critical thresholds.
In collaboration with the Edwards Air Force Research Laboratory, we are investigating computationally the thermal decomposition mechanisms, kinetics, dynamics and product branching of nitrogen-rich explosive compounds, such as dinitrobiuret (DNB), 1-ethyl-3-methylimidazolium dicyanamide, and 1-ethyl-2,3-dimethylimidazolium dicyanamide.

